

Devices used for SCS consist of thin wires placed between the spinal cord and the vertebrae and a small implant placed under the skin in the lower back that helps disrupt pain signals before they can reach the brain. 11 SCS, also known as neurostimulation, has been recommended by doctors for more than 50 years to help people manage chronic pain and improve their quality of life. Pain Foundation, chronic pain is the leading cause of people going to the doctor and costs the nation approximately $635 billion each year in healthcare, disability and lost productivity costs. This makes a big difference in comfort for many patients who now can have access to the best of both worlds – a small, best-in-class rechargeable device with superior stimulation therapy." "Until now, it wasn't available on a rechargeable device that was this small, and that only needs to be charged a few times a year.
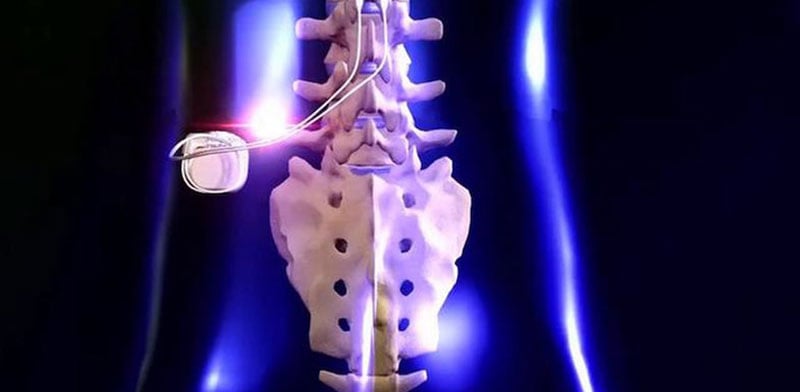
"Abbott's low-dose BurstDR stimulation is clinically proven to reduce pain, improve people's ability to perform everyday activities, and reduce emotional suffering associated with pain," ^8 said Timothy Deer, M.D., DABPM, president and chief executive officer of the Spine and Nerve Centers of the Virginias in Charleston, W.Va. iPhone integration: Abbott's patient-focused mobile app supports real-time battery life and charging status updates of the Eterna SCS system on a personal or Abbott-provided Apple device.U pgradable platform: As Abbott continues to make advancements in SCS therapy, the Eterna SCS system can adapt to future innovations without replacing the implant.MRI capabilities: Eterna uses Abbott's TotalScan™ MRI technology, which allows for full-body MRI scans – a critical need of chronic pain patients who require accessibility to improved diagnostics and healthcare.Lowest recharging requirements: Paired with Abbott's new Xtend™ energy technology, Eterna optimizes the patient charging experience, requiring as few as five recharges per year under standard use from a wireless charger.BurstDR stimulation is preferred to traditional "tingling" tonic stimulation by 87% of patients. Clinically proven therapy: The Eterna SCS system features Abbott's proprietary BurstDR stimulation, which mimics natural firing patterns found in the brain 7 to deliver superior** 8 pain relief.Smallest device: Designed with daily comfort in mind 6 the Eterna SCS system is the smallest implantable, rechargeable spinal cord stimulator* 4 on the market.With patient needs front of mind, Abbott created Eterna to be recharged less than five times a year under normal use, making it the lowest recharge burden platform on the market. The studies found that people wanted a smaller implant for comfort while reducing the need to charge the device daily or weekly. 5Ībbott developed Eterna based on extensive studies with patients, physicians and caregivers to understand the unmet needs of people living with chronic pain. Food and Drug Administration (FDA) approval of the company's Eterna™ spinal cord stimulation (SCS) system – the smallest implantable, rechargeable spinal cord stimulator currently available on the market for the treatment of chronic pain.* 4 Eterna SCS utilizes Abbott's proprietary low-dose BurstDR™ stimulation, the only SCS waveform technology with the highest level of clinical evidence (1A evidence), proven to reduce pain 23% more than traditional waveform technology approaches. 19, 2022 / PRNewswire/ - Abbott (NYSE: ABT) today announced the U.S. This neuromodulation device provides an optimized experience with the ability to wirelessly charge as few as five times per year, the lowest recharge burden compared to other rechargeable SCS systems §1,2,3ĪBBOTT PARK, Ill., Dec.Food and Drug Administration (FDA) approved Abbott's Eterna™ spinal cord stimulation (SCS) system for the treatment of chronic pain


 0 kommentar(er)
0 kommentar(er)
